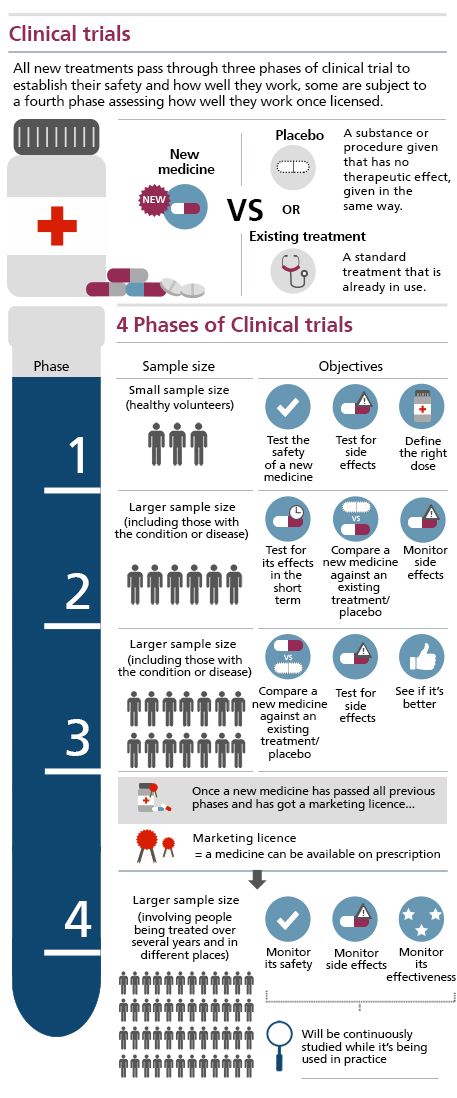
A clinical trial compares the effects of 1 treatment with another. Clinical trials may also be referred to as interventional trials.
Clinical trials are research investigations in which people volunteer to test new treatments interventions or tests as a means to prevent detect treat or manage various diseases or medical conditions.
What is a clinical trial. Clinical trials are research studies that test how well new medical approaches work in people. What are clinical trials and how is a patient eligible to participate. Some investigations look at how people respond to.
Clinical trials are scientific studies conducted to find better ways to prevent screen for diagnose or treat disease. They help find new ways to prevent detect or treat diseases that are safe and effective. Researchers test possible new drugs in the laboratory to begin with.
The purpose of clinical trials is to make sure that these new drugs and medical methods are both safe and effective in people. All new treatments have to be thoroughly tested. Many clinical trials look at new ways to detect diagnose or measure the extent of disease.
About Press Copyright Contact us Creators Advertise Developers Terms Privacy Policy Safety How YouTube works Test new. National Library of Medicine. Clinical trials are medical research studies involving people.
It may involve patients healthy people or both. Clinical trials produce high-quality data for healthcare decision making. Clinical trials are studies involving human subjects that test new treatment methods new drugs and methods for measuring the effectiveness of a test treatment on a disease or medical condition.
How do I take part in a clinical trial. A controlled experiment involving a defined set of human subjects having a clinical event as an outcome measure and intended to yield scientifically valid information about the efficacy or safety of a drug vaccine diagnostic test surgical procedure or other form of medical. The clinical trial template has site lists of libraries for clinical trial protocols protocol documents announcements calendars issues tasks and document discussions.
An adaptive clinical trial is a clinical trial that evaluates a medical device or treatment by observing participant outcomes and possibly other measures such as side-effects on a prescribed schedule and modifying parameters of the trial protocol in accord with those observations. These clinical trials may also show which medical approaches work best for certain illnesses or groups of people. Clinical trials may also compare a new treatment to a treatment that is already available.
ClinicalTrialsgov is a database of privately and publicly funded clinical studies conducted around the world. A clinical trial also known as a clinical research study is a protocol to evaluate the effects and efficacy of experimental medical treatments or behavioral interventions on health outcomes. To download this template you will need access to SharePoint Server 30.
Explore 369503 research studies in all 50 states and in 219 countries. The National Heart Lung and Blood Institute NHLBI leads and supports many studies aimed at preventing diagnosing and treating heart lung blood and sleep disorders. For the purposes of registration a clinical trial is any research study that prospectively assigns human participants or groups of humans to one or more health-related interventions to evaluate the effects on health outcomes.
Some even look at ways to prevent diseases from happening. Clinical trials are medical studies that involve people like you. When carefully conducted they are the safest and fastest way to find new.
Clinical trials are studies to test new drugs already approved drugs devices or other forms of treatments. Clinical trials are research studies performed in people that are aimed at evaluating a medical surgical or behavioral intervention. The adaptation process generally continues throughout the trial as prescribed in the trial protocol.
These can be further customized with different versions of SharePoint. They are the primary way that researchers find out if a new treatment like a new drug or diet or medical device for example a pacemaker is. If they look promising they are carefully tested in people.
Clinical trials are research studies in which people volunteer to help find answers to specific health questions. Each study answers scientific questions and tries to find better ways to prevent screen for diagnose or treat a disease. See listed clinical studies related to the coronavirus disease COVID-19 ClinicalTrialsgov is a resource provided by the US.
You can ask your doctor or a patient organisation if they know of any clinical trials that you may be eligible to join.