O 2 NC 6 H 3 CH 2 OH 2. Negligible Vapour pressure Pa at 100C.
 2 4 6 Trinitrotoluene Solution Sigma Aldrich
2 4 6 Trinitrotoluene Solution Sigma Aldrich
Basis for revised IDLH.
2 4 6 trinitrotoluene. The v lines are all grouped between 800 and 900 kHz. 165 gcm³ Solubility in water g100ml at 20C. 246-Trinitrotoluene TNT CAS No.
246-trinitrotoluene EC Inventory REACH pre-registration EU Ecolabels - Restrictions for Hazardous SubstancesMixtures CL Inventory REACH pre-registration EU Ecolabels - Restrictions for Hazardous SubstancesMixtures. 246-Trinitrotoluene is not expected to volatilize from dry soil surfacesSRC based upon its vapor pressure3. 246-Trinitrotoluene TNT is a yellow odourless unstable solid.
C 7 H 5 N 3 O 6. No inhalation toxicity data are available on which to base an IDLH for 246-trinitrotoluene. The substance identifiers displayed in the InfoCard are the best available substance name EC number CAS number andor the molecular and structural formulas.
Environmental Protection Agency EPA Federal Facilities Restoration and Reuse Office FFRRO provides a summary of 246-trinitrotoluene TNT including its physical and chemical properties. It has been widely used for filling shells grenades and airborne demolition bombs as it is sufficiently insensitive to the shock of ejection from a gun barrel but can be exploded on impact by a detonator mechanism. This yellow solid is occasionally used as a reagent in chemical synthesis but it is best known as an explosive material with convenient handling properties.
This yellow-coloured solid is a reagent reactant in chemistry but is best known as a useful explosive material with convenient handling properties. 0013 Vapour pressure at 20C. And in underwater blasting.
Notice of Rulemaking Title 27 California Code of Regulations Amendment to Section 25705 Specific Regulatory Levels Posing No Significant Risk. 246-Trinitrotoluene TNT a relatively water soluble nitroaromatic compound is a common pollutant in soil and groundwater at sites with substantial military activities such as those associated with munition production handling testing and disposal of explosive and propellent materials. Description Product Name Empirical Formula Hill Notation.
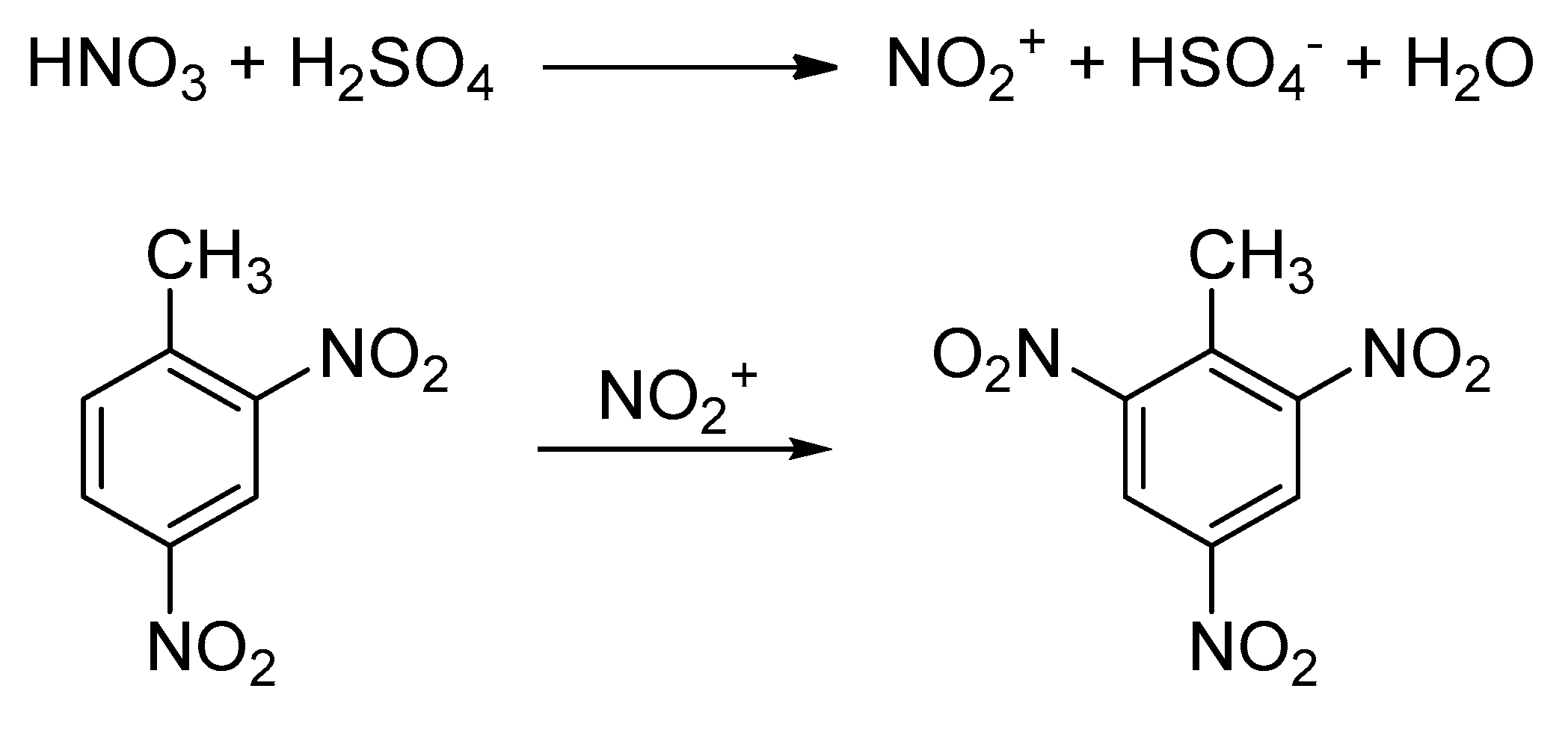
The Substance identity section is calculated from substance identification information from all ECHA databases. 246-Trinitrotoluene Chemicals Listed Effective December 19 2008 As Known To The State Of California To Cause Cancer Or Reproductive Toxicity. Trinitrotoluene TNT or more specifically 246-trinitrotoluene is a chemical compound with the formula C 6 H 2 NO 2 3 CH 3.
This fact sheet developed by the US. 14 Relative vapour density air 1. It is commonly known as TNT and is an explosive used in military shells bombs and grenades in industrial uses and in underwater blasting.
246-Trinitrotoluene is a yellow odorless solid that does not occur naturally in the environment. 246-Trinitrotoluene T3D0082 Trinitrotoluene TNT or more specifically 246-trinitrotoluene is a chemical compound with the formula C6H2 NO23CH3. 1000 μgmL in acetonitrile ampule of 12 mL certified reference material.
Therefore the revised IDLH for 246-trinitrotoluene is 500 mgm 3 based on acute oral toxicity data in humans Deichmann and Gerarde 1969 and animals Dilley et. TNT is an explosive used in military shells bombs and grenades. 785 Octanolwater partition coefficient as log Pow.
2 Product Results Match Criteria. Environmental and health impacts. TNT does not occur naturally in the environment.
C 7 H 5 N 3 O 6 C 6 H 2 CH 3NO 2 3 Molecular mass. 246-Trinitrotoluene TNT November 2017. 246-Trinitrotoluene is used as a high explosive in military and industrial applications.
118-96-7 and Chromium hexavalent compounds. Volatilization of 246-trinitrotoluene from moist soil surfaces is not expected to be an important fate processSRC given an estimated Henrys Law constant of 21X10-8 atm-cu mmoleSRC derived from its vapor pressure of 802X10-6 mm Hg3 and water solubility of 115 mgL4. 2271 Decomposes at 240C Melting point.
The explosive yield of TNT is considered. TNT 246-trinitrotoluene contains three nitrogens per molecule and two molecules per unit cell resulting in six magnetically inequivalent nitrogens.