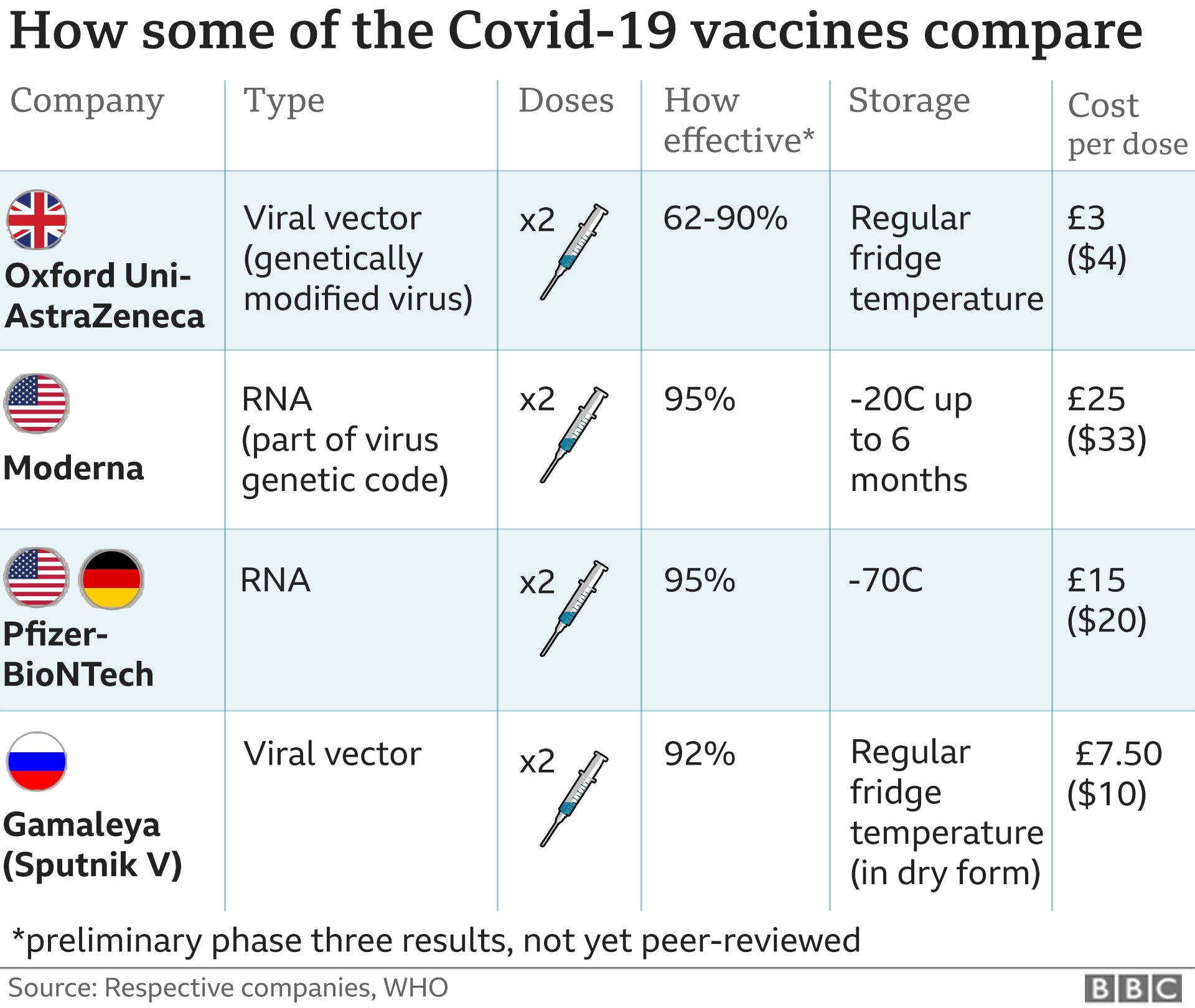
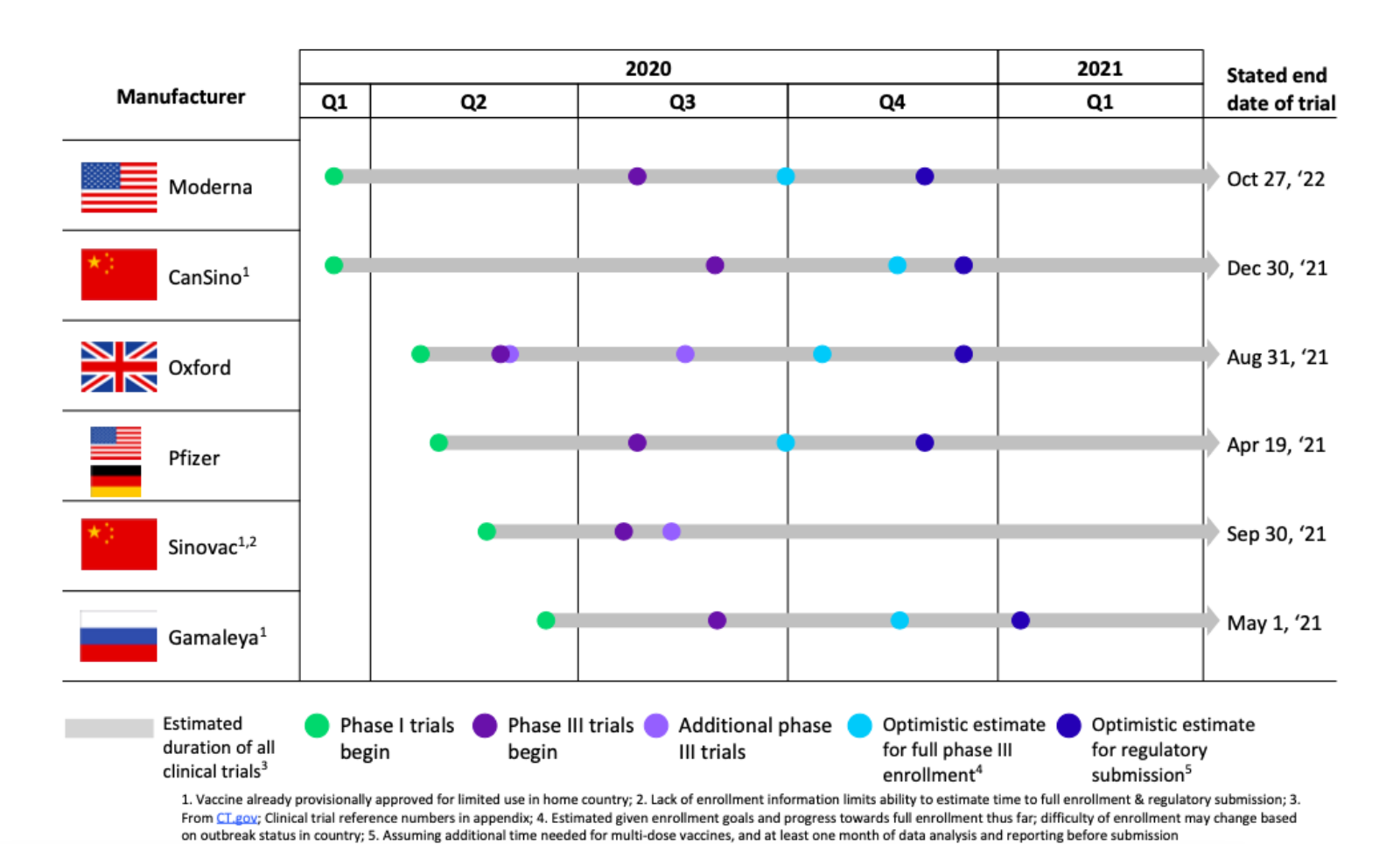
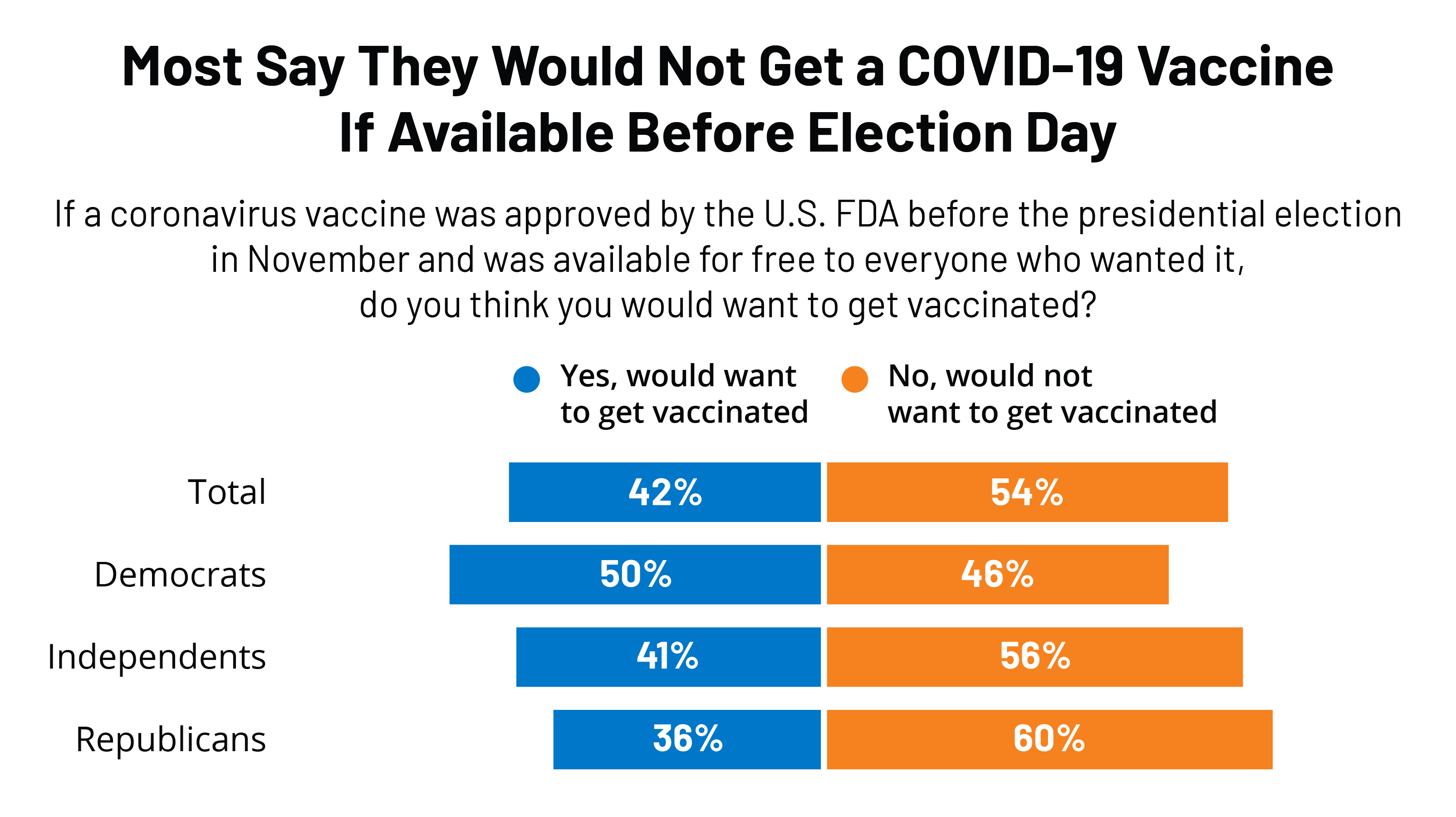
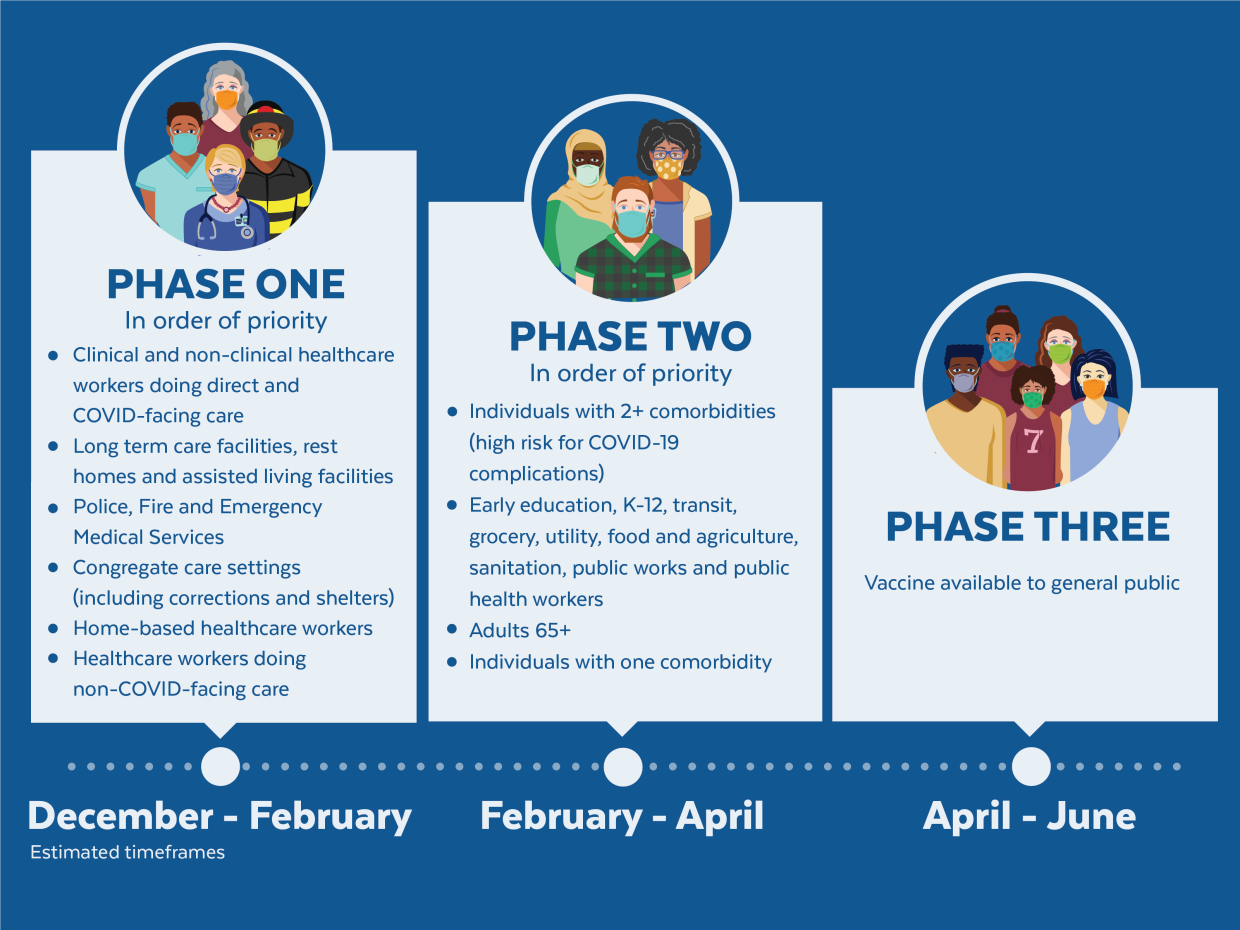
Most women were vaccinated with the MMR vaccine as children but confirm with your doctor or other healthcare professional. There are no controlled data in human pregnancy.
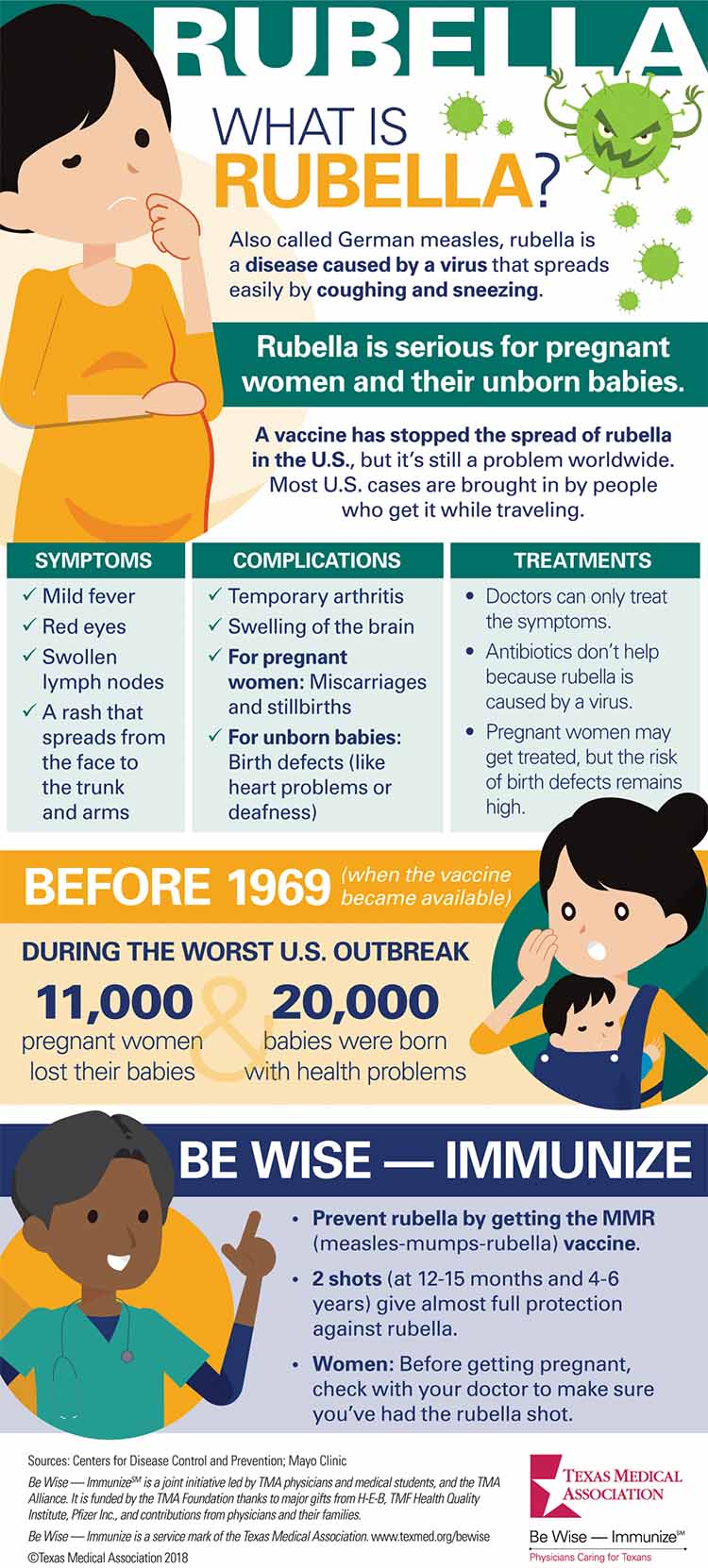 The Virus That Causes Rubella Often Appears Deceptively Mild So Mild In Fact That One Quarter To Half Of People Infected With It Will Have No Symptoms At All According To The
The Virus That Causes Rubella Often Appears Deceptively Mild So Mild In Fact That One Quarter To Half Of People Infected With It Will Have No Symptoms At All According To The
However there are no reports of vaccine-induced congenital rubella syndrome.

Rubella vaccine pregnancy. The MMR vaccine can prevent rubella. Its important to get the measles mumps rubella MMR vaccine at least a month before becoming pregnant in order to protect against rubella during pregnancy which can cause a miscarriage or serious birth defects. Young children who get rubella usually have a mild illness with symptoms that can include a low-grade fever sore throat and a rash that starts on the face and spreads to the rest of the body.
9 This is due to the theoretical risk of transmitting the rubella component of the vaccine to a susceptible fetus. If you are pregnant and have rubella German measles in the first few months of pregnancy there is a high chance that the virus will cause severe damage to your developing baby. I have measles mumps or rubella.
The first rubella virus vaccine is usually given to a child who is 12 to 15 month old. Make sure you have a pre-pregnancy blood test to see if you are immune to the disease. Various attenuated live rubella vaccines now in use are considered generally safe and immunogenic but to date the longest that vaccinal immunity has been shown to endure is seven years.
Measles mumps and rubella MMR vaccine is the best protection against three diseases. Rubella-susceptible women who are not vaccinated because they state they are or may be pregnant should be counseled about the potential risk for CRS and the importance of being vaccinated as soon as they are no longer pregnant. Rubella infection during pregnancy can cause serious health problems for your baby.
Ask at your GP surgery if youre not sure you or your child have had the vaccine. Wait until after your child is born to receive the vaccine. Make sure you and your child are protected from rubella by getting vaccinated on schedule.
Others living in the home should be vaccinated. Vaccinated women should avoid pregnancy for 28 days after vaccination. The best ways to protect yourself against measles mumps and rubella are to avoid others who are sick with these diseases wash your hands with soap and water and to get vaccinated before becoming pregnant.
Subclinical reinfection is not uncommon among vaccines but its effect on pregnancy and fetal development is not. The MMR vaccine given before pregnancy provides protection against rubella infection in any future pregnanciesThe MMR is a live vaccine and must be given at least one month before pregnancy. This will help protect both mother and her baby.
It also protects you from measles and mumps. The MMR vaccine is offered to all children in the UK. MMR or varicella vaccination during pregnancy should not be considered a reason to terminate pregnancy.
If you are not already vaccinated against rubella you should be vaccinated before you get pregnant. Vaccine in pregnancy surveillance was established in 1981 specifically for rubella vaccine originally under the. The virus affects the developing organs and the baby may be born with serious disability - this is.
The Rubella virus strain RA 273 was obtained from an infected aborted fetus3 Since the 60s this virus strain has been used as the chief component of the Rubella vaccine. Abnormalities suggestive of congenital rubella syndrome were not observed during a 10-year survey of 700 pregnant women who received rubella. There is a risk of viral transmission to the fetus.
2 doses can give lifelong protection against measles mumps and rubella. You should not receive a rubella virus vaccine if you are pregnant. Meningococcal MenACWY or MPSV4.
Animal studies have not been reported. Visit the Rubella immunisation service page for information on receiving the rubella vaccine. Rubella vaccine has been assigned to pregnancy category C by the FDA.
Rubella virus vaccine Pregnancy Warnings. Avoid becoming pregnant for at least 3 months after receiving a rubella virus vaccine. The MMR vaccine provides immunity to infection from Rubella.
PHE guidance recommends that when rubella is suspected pregnant women should be simultaneously investigated for immunity to both rubella and parvovirus B19 unless their immune status is already known PHE 2015b. The best protection against rubella is the MMR measles-mumps-rubella vaccine. For some peopleespecially pregnant women and their unborn babiesrubella can be serious.
If you arent up to date with the MMR vaccine youll need it before you get pregnant. During the 1964 Rubella epidemic many women were advised to abort babies if the mothers contracted Rubella during pregnancy. MMR vaccine is not recommended in pregnancy as matter of caution.